




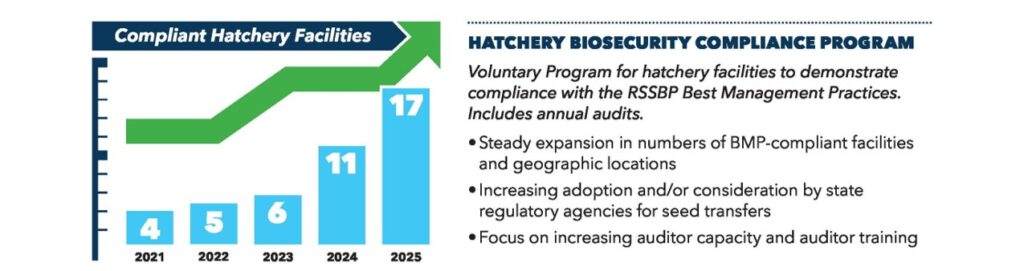
Hatchery Compliance Program
GOAL
Incentivize best management practices for biosecurity during the production and transfer of shellfish seed to streamline the facilitation of regulation and commerce.
PREMISE
The most biosecure shellfish products are those that have not been exposed to raw water, namely larvae and newly set seed. Even small seed that have low filtration rates and less exposure to untreated seawater are more biosecure than larger, older seed.
- Safi, L.S.L., McGurk, E. S., Carnegie, R.B., and Bushek, D. (2025 online/in press) The biosecurity of bivalve seed transfers during different life stages with respect to their pathogens. Aquaculture. 606 article 742548. https://doi.org/10.1016/j.aquaculture.2025.742582
PARTICIPATION IS VOLUNTARY
HATCHERIES are encouraged to apply regardless if participating in seed transfers.
Adopt RSSBP best management practices (BMPs) to improve the facility biosecurity.

STATE REGULATORS are encouraged to use the RSSBP tools to evaluate seed import requests.
- RSSBP provides tools to assist in the decision-making process as it relates to shellfish disease risk (one of the import considerations).
RSSBP BEST MANAGEMENT PRACTICES
The BMPs are simple foundations of biosecurity that should be considered whether or not your hatchery facility participates in seed transfers or sales. Implementation will look different based on a number of factors such as facility size, floor plan, and business model. RSSBP welcomes facilities of all sizes and levels of sophistication. Please contact us if you have any questions about participation.
Find a detailed list of BMPs here.
Check out the different ways facilities may address the BMPs here
Water Treatment
Water treatment to prevent pathogen exposure during early life stage cultivation is required (1um or other demonstrated means).
Separation
Adequate separation of untreated and treated water areas to avoid cross contamination is required.
Record Keeping
Records should be kept for broodstock, spawning, and maintenance of systems used to eliminate POCs.
Heath Exams
Health examinations should be conducted on animals experiencing unexplained, atypical mortality.
Regulatory Compliance
Facilities must be compliant with all Federal, State and Local permitting requirements.
APPLICATION
PARTICIPATION STEPS
Enrolled Facility
Hatchery practices listed on application are aligned with Program BMPs.
Listed as participant
BMP-Compliant Facility
Pass an annual, in-person facility audit which verifies the biosecurity practices are in place.
Listed as participant + Receive letter of compliance
Biosecure Product(s)
Required 3-year health history (specific to species and size) is in place. Products must be maintained on 1 um treated water (or other means) until transferred.
FACILITY AUDIT DETAILS
The audit process does not negatively impact industry commerce. It is an opportunity for dialog and to find ways to further improve biosecurity.
How/When?
Audits are set up by a member of the RSSBP team and generally held in the fall to early spring depending on the facility operating schedule. Ideally, auditors will be onsite to see active operation, but will avoid peak production times.
At present, there is no cost to the hatchery for an audit.
Who?
The auditors are independent of the RSSBP; selected to maintain consistency across facilities and remove any perceived bias. Initial audits are conducted by at least two auditors to provide a comprehensive review of the BMP implementation.
What?
The audit consists of a detailed walkthrough of the facility.
Audits typically `follow the water’ starting where the source water enters, is
treated, and is distributed to other areas. An initial audit takes close to two hours, depending on the size of the facility and complexity of the systems.
Result
Auditors provide a recommendation to approve, deny, or conditionally approve
(pending a corrective action) facility BMP compliance. The Advisory Council (minus industry members) review, resolve any concerns, and make a final decision, within a few weeks of the audit. Hatcheries will receive a letter of compliance to share with state regulators and are listed on rssbp.org as a participating hatchery.
BIOSECURE PRODUCT REQUIREMENTS
Eligible Products must be held on water treated to eliminate pathogens (e.g., 1µm filtered water or well water). At this time, nursery products reared in untreated, ambient waters are not eligible.
Eligible Products must have records of health evaluations from an independent pathology laboratory that:
- cover the previous three years;
- with a minimum of two sampling events per species, per year during the production season (6 samples over the 3- year period);
- that demonstrate no detections of POCs. The sole exception is an acceptable level of Perkinsus marinus (Dermo disease) where it is ubiquitously distributed and persistent.
